18+ Molality Calculation
Web Next we need to convert the given mass to moles. The calculators are numbered because sometimes the results of one calculator.
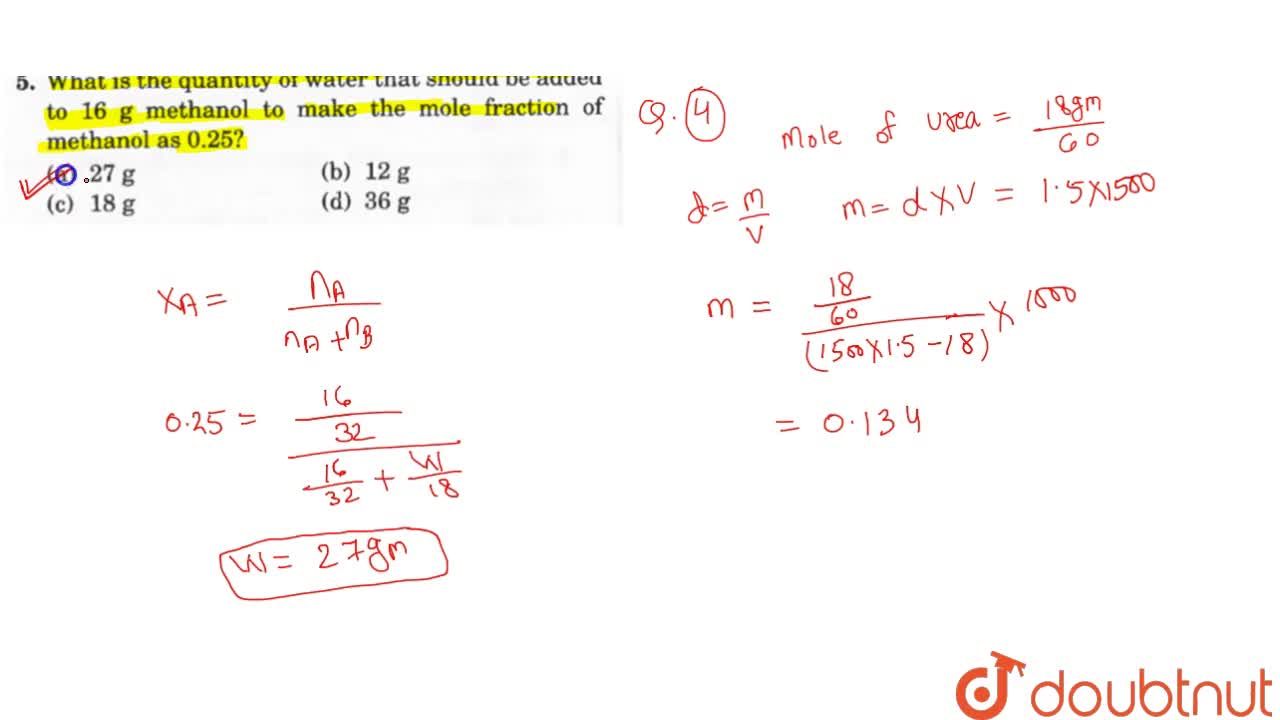
Molality It Is Defined As The Moles Of The Solute Pressent In 1 Kg Of The Solvent It Is Denoted By M Molality M Number Of Moles Of Solute Number Of Kilograms Of
D MW 10 Molarity Where.

. 5 g x 1 mol 9521 g 00525 mol. Web Molarity calculations Google Classroom You might need. Expressing the volume in.
Web So the molar mass of H 2 O is 2 x 1008 159994 180154 gmol. If a solution of salt. Molality m Moles of SoluteKilograms of Solvent Our free molality calculator also makes use of the same.
Web Liters of solution mL of solution x 1 L1000 mL Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L. Web The Tocris molarity calculator is a useful tool which allows you to calculate the. Web The following equation is used for calculating acid and base molarity where the concentration is given in wt.
Web Molality is a measurement of the concentration of a solution by comparing the moles of the solute with the kilograms of the solvent the solute is dissolved in. Molality similar to molarity however calculation of molality uses the mass rather than volume of the. The solvent water mass has to be in kilograms so we have 05 kg of water.
Web molality calculation 510 moles 0375 kilograms 136 m 3 sig figs In this problem the moles of sodium chloride are calculated from the grams by using the molar. Now the number of moles of NaCl is given as follows. Web The procedure to use the molality calculator is as follows.
This means molality does not change with a. Web 1120 g soln 300mol NaCl 584 g 1 mol NaCl 1120 g soln 175 g NaCl 945 g water 0945 kg of water Now use the units of molality as an equation. Calculator A 0674text M 0674M cobalt II chloride text CoCl_2 CoCl2 solution is prepared with a total volume.
The calculators on this page are independent and can be used in any order. Mass of a compound required to prepare a solution of known volume and concentration volume of. Enter the number of moles of solute and weight of solvent x for the unknown value in the input field Step 2.
Weight. Web 18 g mol. To dilute a solution of known molarity please use the.
Web The molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume. Web You can calculate molality by using the following molality formula. Number of Moles Given mass of NaCl Molar Mass of NaCl 12 585 00205 mol.
Web As molality is not dependent on the volume of the solution it is independent of pressure and temperature changes. This is enough to calculate the molarity.

Experimental Data And Model Fit For The Formation Constant Of Am Cit 2 Download Scientific Diagram

Molality Formula Examples How To Calculate Molality Video Lesson Transcript Study Com

Molality Formula Examples How To Calculate Molality Video Lesson Transcript Study Com

Molality Formula Examples How To Calculate Molality Video Lesson Transcript Study Com

Sk015 Flip Ebook Pages 1 50 Anyflip

Chapter 1 Matter Flip Ebook Pages 1 50 Anyflip
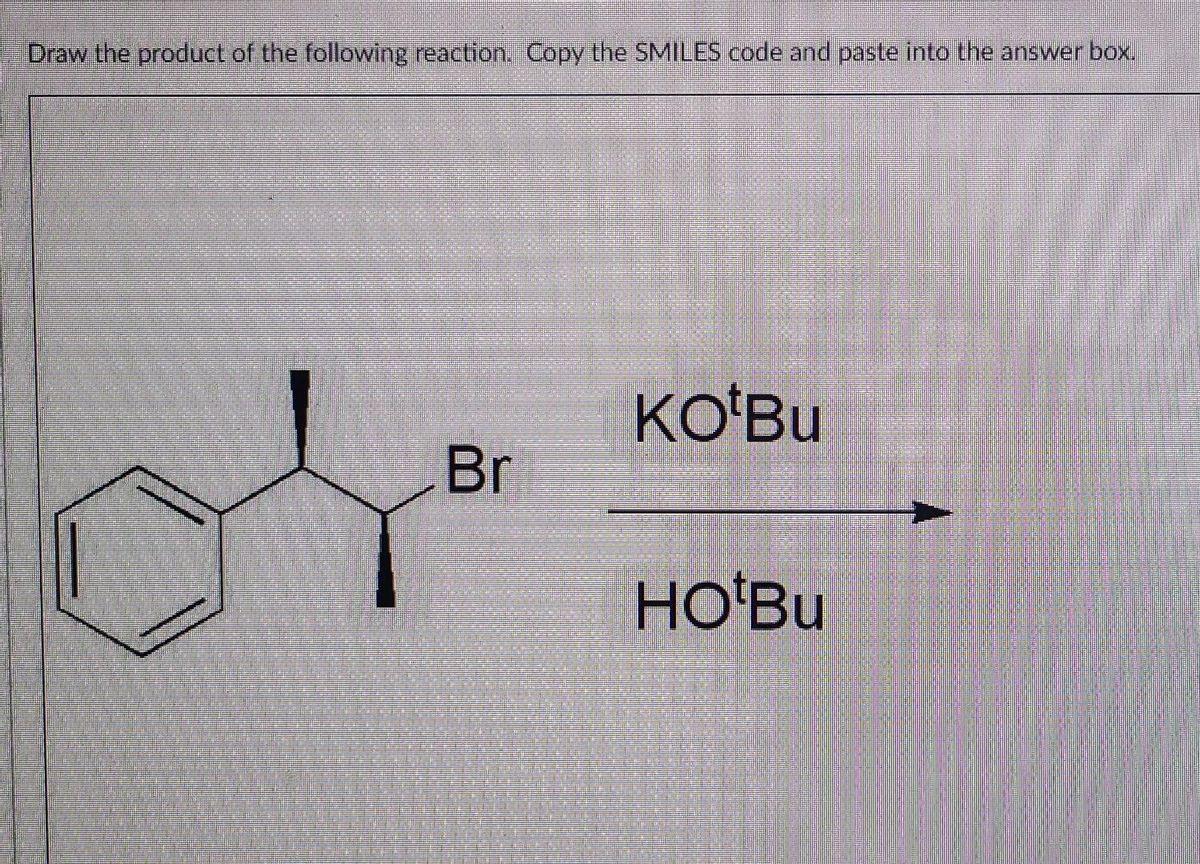
Answered Br Ko Bu Hotbu Bartleby

Chem Team Molality Problems 1 10 Molality Problems 1 Go To Molality Problems 11 Return To The Studocu
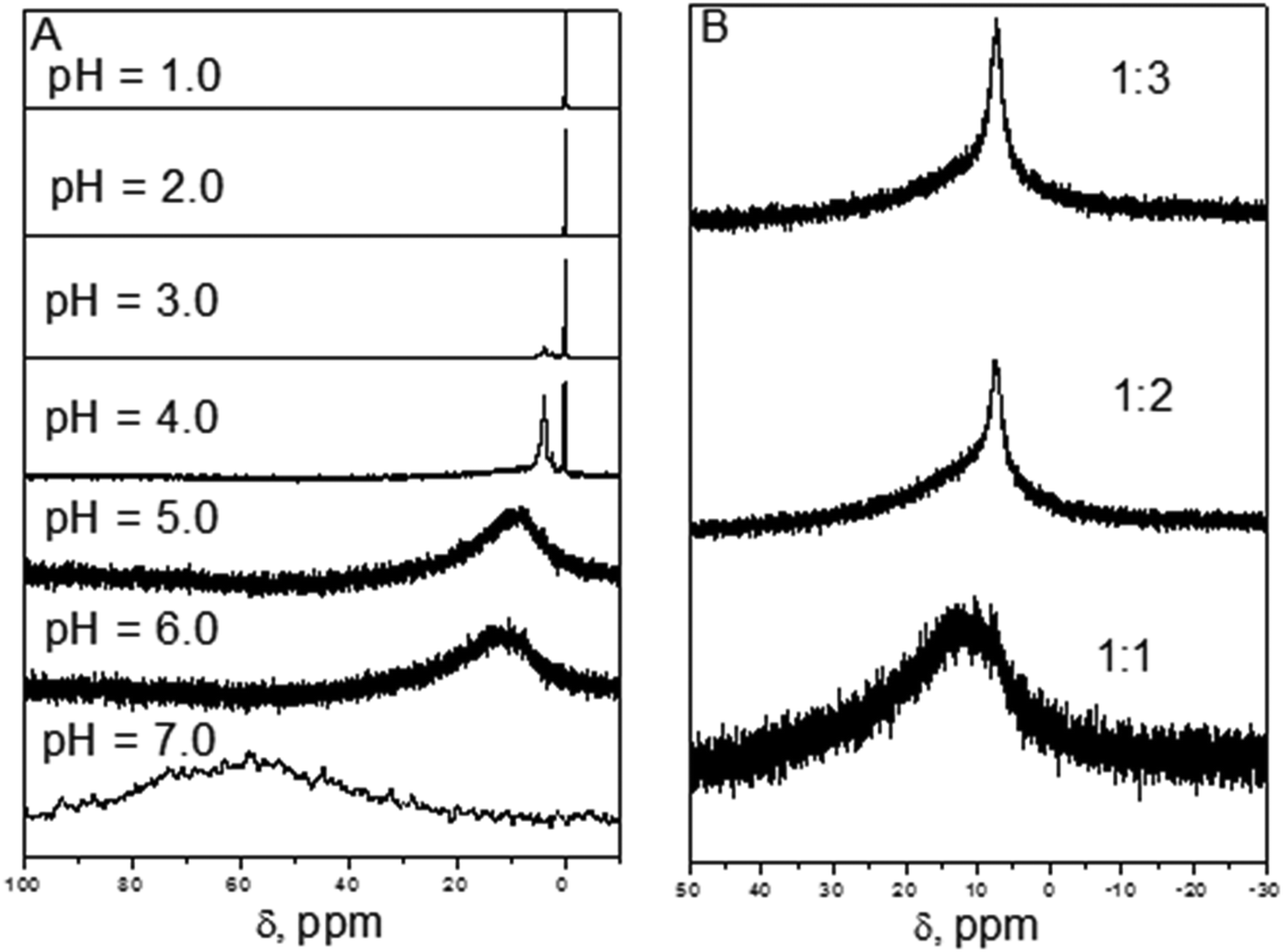
Stabilization Of Al Iii Solutions By Complexation With Cacodylic Acid Speciation And Binding Features Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C5cp04717j

Alcohol Reverses The Effects Of Kcnj6 Girk2 Noncoding Variants On Excitability Of Human Glutamatergic Neurons Biorxiv

Chapter 1 Matter Flip Ebook Pages 1 50 Anyflip

The Molality M Of Each Ion Nh4 And Cl Respectively Present In The Aqueous Solution Of 2m Nh4cl Assuming 100 Dissociation According To Given Reaction Is Nh4cl Aq

Calculations Pdf Nitric Acid Sodium Bicarbonate

Pdf Predictors Of Altered Sensorium At Admission In Children With Diabetic Ketoacidosis

Molality Formula Examples How To Calculate Molality Video Lesson Transcript Study Com

Clinical Chemistry Powerpoint Slides

Stabilization Of Al Iii Solutions By Complexation With Cacodylic Acid Speciation And Binding Features Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C5cp04717j